History of the atom worksheet answers – Embark on a journey through the history of the atom, a tale that has shaped our understanding of the universe. From Democritus’s early atomic theory to the groundbreaking discoveries of Thomson, Rutherford, and Bohr, we unravel the fundamental building blocks of matter and explore their profound impact on science and technology.
As we delve into the evolution of atomic theory, we witness the remarkable contributions of scientists who have dedicated their lives to unlocking the secrets of the atom. Their tireless efforts have laid the foundation for modern physics and chemistry, revolutionizing our understanding of the world around us.
Introduction to the History of the Atom
The atom is the basic unit of matter and the fundamental building block of all chemical elements. It consists of a nucleus, which contains protons and neutrons, and electrons that orbit the nucleus.
The concept of the atom has evolved over time, with significant contributions from various scientists throughout history. The first major breakthrough came in the 18th century when John Dalton proposed the atomic theory, which laid the foundation for our modern understanding of the atom.
Dalton’s Atomic Theory
- Matter is composed of indivisible particles called atoms.
- All atoms of the same element are identical in mass and other properties.
- Atoms of different elements have different masses and properties.
- Chemical reactions involve the rearrangement of atoms, not their creation or destruction.
Dalton’s theory provided a solid foundation for the study of chemistry, but it was not until the early 20th century that scientists began to unravel the internal structure of the atom.
Democritus and the Early Atomic Theory
Democritus, a Greek philosopher who lived in the 5th century BC, is widely recognized as the father of the atomic theory. His groundbreaking ideas laid the foundation for our modern understanding of the structure of matter.
Democritus proposed that all matter is composed of indivisible, indestructible particles called atoms. He believed that these atoms were in constant motion and could combine in different ways to form different substances.
Key Ideas of Democritus’s Atomic Theory
- Indivisibility:Democritus believed that atoms were the smallest possible units of matter and could not be further divided.
- Motion:He proposed that atoms were constantly moving and colliding with each other.
- Void:Democritus recognized the existence of a void, or empty space, between atoms.
- Variety:He believed that atoms existed in different shapes and sizes, accounting for the diversity of matter.
- Changelessness:Democritus asserted that atoms were immutable and could not be created or destroyed.
Dalton’s Atomic Theory
John Dalton’s atomic theory, proposed in the early 19th century, laid the foundation for modern chemistry. It marked a significant advancement in the understanding of atomic structure and laid the groundwork for further discoveries.
Dalton’s theory consisted of several key postulates:
- Matter is composed of extremely small, indivisible particles called atoms.
- All atoms of a given element are identical in size, mass, and other properties.
- Atoms of different elements differ in size, mass, and other properties.
- Atoms cannot be created or destroyed.
- Atoms combine in simple whole-number ratios to form compounds.
Dalton’s theory revolutionized the understanding of atomic structure. It provided a framework for explaining the properties of elements and compounds and paved the way for further developments in chemistry.
Thomson’s Discovery of Electrons
In 1897, English physicist J.J. Thomson conducted a series of experiments that revolutionized our understanding of the atom. His experiments provided the first evidence for the existence of electrons, the fundamental particles that make up all matter.
Thomson’s experiments involved passing an electric current through a vacuum tube. He observed that a stream of negatively charged particles was emitted from the cathode, the negative electrode. These particles were named electrons by Thomson.
Thomson’s discovery of electrons had profound implications for the atomic model. It showed that atoms were not indivisible particles, as had been previously believed, but rather were composed of even smaller particles. This discovery paved the way for the development of the modern atomic model.
Thomson’s Experiment
Thomson’s experiment consisted of passing an electric current through a vacuum tube. The vacuum tube was a sealed glass tube from which most of the air had been removed. The tube contained two electrodes, a cathode and an anode. The cathode was a negatively charged metal plate, and the anode was a positively charged metal plate.
When an electric current was passed through the vacuum tube, a stream of negatively charged particles was emitted from the cathode. These particles were attracted to the positively charged anode, and they traveled through the vacuum tube in a straight line.
Thomson measured the speed and charge of the particles emitted from the cathode. He found that the particles were very small and that they had a negative charge. He also found that the particles were all identical, regardless of the material of the cathode.
Rutherford’s Gold Foil Experiment

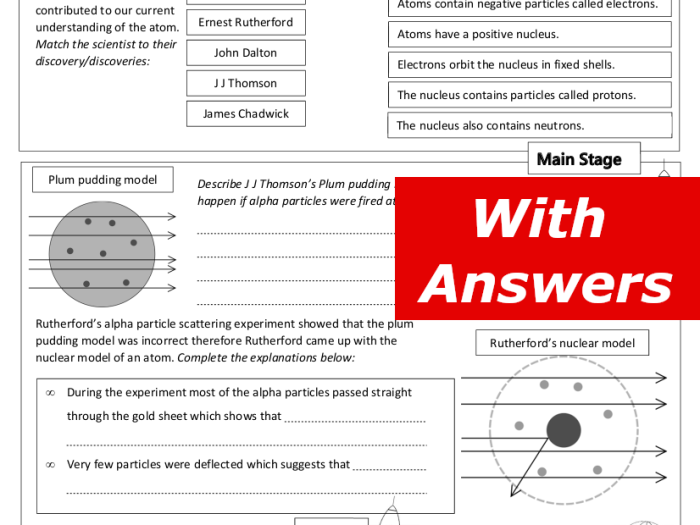
Ernest Rutherford’s gold foil experiment was a groundbreaking experiment in nuclear physics that took place in 1911. It provided crucial evidence for the nuclear model of the atom, which proposed that the atom has a small, dense nucleus surrounded by orbiting electrons.Rutherford’s
experiment involved firing a beam of alpha particles (helium nuclei) at a thin sheet of gold foil. He expected most of the alpha particles to pass straight through the foil, as atoms were believed to be mostly empty space. However, to his surprise, a significant number of alpha particles were deflected by the foil, some even rebounding back towards the source.Rutherford’s
experiment demonstrated that the atom has a small, dense nucleus containing most of the atom’s mass. The nucleus is surrounded by a much larger region of empty space where the electrons orbit. This discovery revolutionized our understanding of the atom and laid the foundation for modern atomic physics.
Significance of Rutherford’s Experiment:
- Provided evidence for the nuclear model of the atom, which is the accepted model today.
- Established that the nucleus is extremely small compared to the size of the atom.
- Led to the development of the planetary model of the atom, where electrons orbit the nucleus in circular paths.
- Contributed to the understanding of the structure of elements and the periodic table.
Bohr’s Atomic Model
Niels Bohr, a Danish physicist, proposed an atomic model in 1913 that revolutionized the understanding of atomic structure. His model addressed the limitations of previous models, such as the inability to explain the stability of atoms and the emission of discrete wavelengths of light by atoms.
Bohr’s model introduced the concept of energy levels, which are specific energy states that electrons can occupy within an atom. Electrons can transition between these energy levels by absorbing or emitting photons of light with energy equal to the difference in energy between the levels.
Key Features of Bohr’s Atomic Model, History of the atom worksheet answers
- Atoms have a central, positively charged nucleus containing protons and neutrons.
- Electrons orbit the nucleus in discrete, circular energy levels.
- Electrons can transition between energy levels by absorbing or emitting photons of light.
- The energy of each energy level is quantized, meaning it can only take on certain specific values.
How Bohr’s Model Addressed Limitations of Previous Models
- Bohr’s model explained the stability of atoms by introducing the concept of energy levels. Electrons in the lowest energy level are most stable and do not emit radiation.
- The model also explained the emission of discrete wavelengths of light by atoms. When electrons transition between energy levels, they emit photons of light with energy equal to the difference in energy between the levels.
Quantum Mechanics and the Modern Atomic Model
Quantum mechanics, developed in the early 20th century, revolutionized the understanding of the atom. It introduced the concept of wave-particle duality, where particles like electrons can also behave like waves, and the quantization of energy, where energy can only exist in discrete levels.
These concepts led to the development of the modern atomic model, which describes the atom as a central nucleus surrounded by electrons occupying specific energy levels. The nucleus contains protons and neutrons, and the electrons are arranged in shells around the nucleus.
Key Concepts of Quantum Mechanics
- Wave-particle duality:Matter can exhibit both particle-like and wave-like properties.
- Quantization of energy:Energy can only exist in discrete levels, called energy levels or quantum states.
- Uncertainty principle:It is impossible to simultaneously know both the position and momentum of a particle with perfect accuracy.
Isotopes and the Atomic Number
Isotopes are variations of an element that have the same number of protons but differ in the number of neutrons. The atomic number of an element is determined by the number of protons in its nucleus. Since isotopes have the same number of protons, they have the same atomic number and belong to the same element.
The difference in the number of neutrons affects the mass of the isotope. Isotopes with more neutrons are heavier than those with fewer neutrons. Isotopes are represented by the element symbol followed by a superscript indicating the mass number, which is the sum of the number of protons and neutrons in the nucleus.
Examples of Isotopes and their Applications
- Carbon-12 (12C) : The most common isotope of carbon, used as a reference for atomic mass.
- Carbon-14 (14C) : A radioactive isotope used in carbon dating to determine the age of organic materials.
- Uranium-235 (235U) : A fissile isotope used in nuclear reactors and weapons.
- Uranium-238 (238U) : A non-fissile isotope used as fuel in nuclear reactors.
- Hydrogen-1 (1H) : The most common isotope of hydrogen, also known as protium.
- Hydrogen-2 (2H) : Also known as deuterium, used in nuclear fusion reactions.
- Hydrogen-3 (3H) : Also known as tritium, a radioactive isotope used in nuclear weapons and fusion reactors.
Applications of Atomic Theory
The understanding of atoms has revolutionized various fields, leading to advancements in technology and scientific research.
The practical applications of atomic theory include:
Nuclear Energy
Nuclear power plants harness the energy released from atomic reactions, providing a significant source of electricity worldwide.
Medical Applications
- Radiotherapy:Ionizing radiation from radioactive isotopes is used to treat cancer cells.
- Medical Imaging:Techniques like X-rays and magnetic resonance imaging (MRI) utilize atomic properties to produce images of internal body structures.
- Radioisotopes in Medicine:Radioactive isotopes are used in diagnostic tests and treatments, such as PET scans and thyroid function tests.
Materials Science
The understanding of atomic interactions has led to the development of new materials with tailored properties, such as:
- Nanotechnology:Manipulating atoms and molecules at the nanoscale has enabled the creation of advanced materials for electronics, medicine, and energy storage.
- Semiconductors:The controlled manipulation of electrons in semiconductors forms the basis of modern electronics, including transistors, integrated circuits, and computer chips.
Environmental Science
Atomic theory plays a crucial role in understanding environmental issues, such as:
- Nuclear Waste Disposal:The safe storage and disposal of radioactive waste from nuclear power plants require knowledge of atomic properties and radiation effects.
- Radioactive Dating:By measuring the decay of radioactive isotopes, scientists can determine the age of archaeological artifacts, fossils, and geological formations.
Other Applications
- Atomic Clocks:The highly precise and stable atomic transitions provide the basis for accurate timekeeping.
- Laser Technology:The manipulation of atomic energy levels enables the production of lasers, which have numerous applications in medicine, industry, and research.
FAQ Summary: History Of The Atom Worksheet Answers
What is the basic structure of an atom?
An atom consists of a nucleus, which contains protons and neutrons, surrounded by a cloud of electrons.
Who is credited with the first atomic theory?
Democritus, a Greek philosopher, first proposed the idea of atoms around 400 BC.
What did John Dalton’s atomic theory contribute to our understanding of atoms?
Dalton’s theory introduced the concepts of indivisible atoms, constant composition of elements, and multiple proportions.