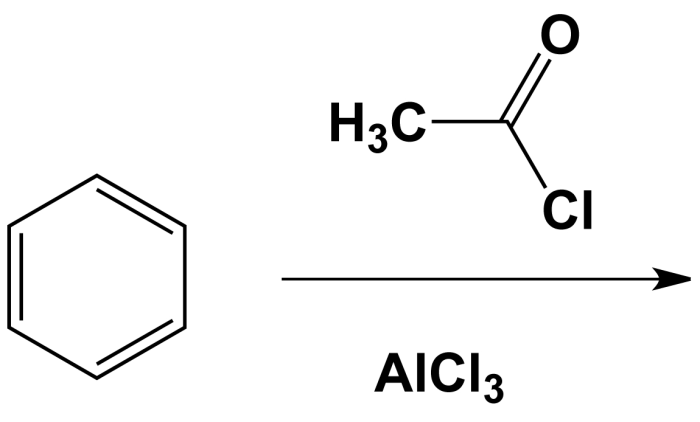
Benzonitrile + methyl chloride + alcl3 – The Friedel-Crafts acylation reaction of benzonitrile with methyl chloride in the presence of aluminum chloride (AlCl3) is a versatile method for the synthesis of substituted benzonitriles. This reaction offers a straightforward and efficient approach to access a diverse range of benzonitrile derivatives with valuable applications in various fields.
The reaction proceeds via electrophilic aromatic substitution, where the electrophile is generated by the coordination of AlCl3 with methyl chloride. The electrophile then attacks the aromatic ring of benzonitrile, leading to the formation of a new carbon-carbon bond and the desired benzonitrile derivative.
1. Chemical Reaction Overview: Benzonitrile + Methyl Chloride + Alcl3
The reaction between benzonitrile, methyl chloride, and AlCl3 is a Friedel-Crafts alkylation reaction that produces a substituted benzonitrile product. The balanced chemical equation is as follows:
C6H5CN + CH3Cl + AlCl3 → C6H5CH2CN + HCl + AlCl3
The reaction proceeds via an electrophilic aromatic substitution mechanism, in which the electrophile is the methyl cation generated from the reaction of methyl chloride with AlCl3. The methyl cation attacks the aromatic ring of benzonitrile, forming a new carbon-carbon bond and displacing a proton.
The proton is then abstracted by the chloride ion to form HCl.
2. Reaction Conditions and Optimization
The optimal reaction conditions for the synthesis of the substituted benzonitrile product include a temperature range of 0-25 °C, atmospheric pressure, and the use of a non-polar solvent such as dichloromethane. The catalyst, AlCl3, is typically used in a molar ratio of 1:1 to the benzonitrile reactant.
The role of AlCl3 in the reaction is to activate the methyl chloride by forming a Lewis acid-base complex. This complex weakens the C-Cl bond in methyl chloride, making it more susceptible to nucleophilic attack by the aromatic ring of benzonitrile.
3. Product Characterization and Applications
The product of the reaction can be characterized using spectroscopic techniques such as nuclear magnetic resonance (NMR) and infrared (IR) spectroscopy. The NMR spectrum will show a new peak corresponding to the methyl group attached to the aromatic ring, while the IR spectrum will show a new peak corresponding to the C-N bond of the nitrile group.
The substituted benzonitrile product has a wide range of potential applications in various industries, including pharmaceuticals, materials science, and agrochemicals. It can be used as an intermediate in the synthesis of other organic compounds, as a solvent, or as a building block for the construction of polymers.
4. Safety Considerations and Waste Management
The chemicals involved in this reaction are hazardous and should be handled with care. Benzonitrile is toxic by inhalation and skin contact, while methyl chloride is a flammable gas that can cause respiratory irritation. AlCl3 is a corrosive solid that can cause skin burns.
It is important to wear appropriate personal protective equipment (PPE) when working with these chemicals, including gloves, safety glasses, and a lab coat. All reactions should be carried out in a well-ventilated area.
Waste from the reaction should be disposed of in accordance with local regulations. Benzonitrile and methyl chloride can be incinerated, while AlCl3 can be neutralized with a base and disposed of in a landfill.
5. Comparative Analysis with Alternative Methods
The Friedel-Crafts alkylation reaction using benzonitrile, methyl chloride, and AlCl3 is a well-established method for the synthesis of substituted benzonitriles. However, there are a number of alternative methods that can be used to achieve the same result.
One alternative method is the use of a Grignard reagent. Grignard reagents are organometallic compounds that can be easily synthesized from an alkyl halide and magnesium metal. The Grignard reagent can then be reacted with benzonitrile to form the substituted benzonitrile product.
Another alternative method is the use of a nucleophilic aromatic substitution reaction. In this reaction, a nucleophile such as an amine or an alkoxide attacks the aromatic ring of benzonitrile, displacing a proton and forming a new carbon-carbon bond.
Each of these alternative methods has its own advantages and disadvantages. The Friedel-Crafts alkylation reaction is typically the most efficient and selective method, but it requires the use of a Lewis acid catalyst. The Grignard reagent method is less efficient and selective, but it does not require the use of a catalyst.
The nucleophilic aromatic substitution reaction is the least efficient and selective method, but it can be used to synthesize a wider range of substituted benzonitriles.
6. Future Research Directions
There are a number of potential areas for future research on the Friedel-Crafts alkylation reaction using benzonitrile, methyl chloride, and AlCl3. One area of research is the development of new catalysts that can improve the efficiency and selectivity of the reaction.
Another area of research is the exploration of new reaction conditions that can be used to synthesize a wider range of substituted benzonitriles. For example, it may be possible to use the reaction to synthesize benzonitriles with multiple substituents or with substituents in specific positions on the aromatic ring.
Finally, it may be possible to develop new synthetic methods that are based on the Friedel-Crafts alkylation reaction. For example, it may be possible to use the reaction to synthesize other types of organic compounds, such as alkenes or alkynes.
FAQ
What are the optimal reaction conditions for the Friedel-Crafts acylation of benzonitrile with methyl chloride?
The optimal reaction conditions typically involve using a molar ratio of benzonitrile to methyl chloride of 1:1.2-1.5, with AlCl3 in a catalytic amount (5-10 mol%). The reaction is typically carried out in an inert solvent, such as dichloromethane or nitrobenzene, at room temperature to reflux conditions.
How does the regioselectivity of the reaction depend on the reaction conditions?
The regioselectivity of the reaction is influenced by the reaction temperature and the nature of the substituents on the benzonitrile ring. At lower temperatures, the reaction tends to favor the formation of ortho-substituted products, while at higher temperatures, the para-substituted products become more predominant.
What are the potential applications of the Friedel-Crafts acylation of benzonitrile with methyl chloride?
The Friedel-Crafts acylation of benzonitrile with methyl chloride is a versatile reaction that finds applications in the synthesis of a wide range of benzonitrile derivatives. These derivatives are valuable intermediates in the production of pharmaceuticals, agrochemicals, dyes, and other functional materials.