Unit 6 worksheet 4 molecular compounds – Embarking on Unit 6 Worksheet 4: Molecular Compounds, we embark on a captivating journey into the fascinating world of chemistry. Molecular compounds, the building blocks of our universe, hold immense significance in various scientific disciplines and everyday applications. Prepare to unravel their intricate nature, delve into their unique properties, and explore their diverse roles in shaping our world.
As we delve deeper into the intricacies of molecular compounds, we will decipher the rules governing their nomenclature, unravel the secrets behind their physical and chemical properties, and witness their remarkable applications in fields ranging from medicine to materials science.
Brace yourselves for an enlightening exploration that promises to expand your understanding of the molecular realm.
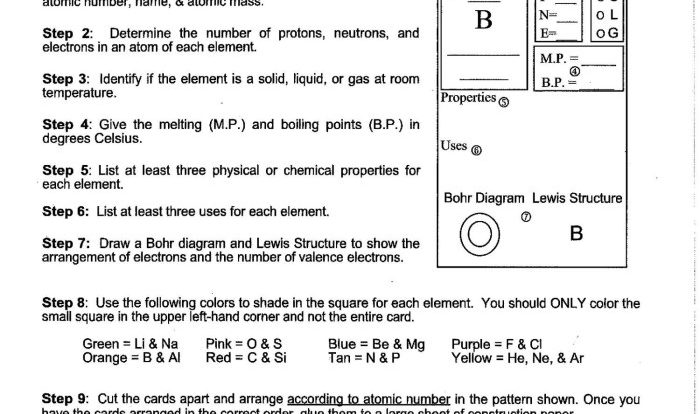
1. Introduction to Molecular Compounds: Unit 6 Worksheet 4 Molecular Compounds
Molecular compounds are chemical compounds composed of two or more non-metallic elements that are held together by covalent bonds. They are formed when atoms share electrons to achieve a stable electron configuration. Molecular compounds typically have lower melting and boiling points compared to ionic compounds and are generally poor conductors of electricity.
The formation of molecular compounds can be explained by the Lewis dot structure, which shows the valence electrons of each atom involved. When atoms share electrons to form a covalent bond, they achieve a more stable electron configuration and a lower energy state.
2. Nomenclature of Molecular Compounds
Binary molecular compounds are named using prefixes that indicate the number of atoms of each element present in the molecule. For example, CO2 is carbon dioxide, where the prefix “mono” is omitted for the first element.
For larger molecules, Greek prefixes are used to indicate the number of atoms. For example, N2O5 is dinitrogen pentoxide, where “di” indicates two nitrogen atoms and “penta” indicates five oxygen atoms.
Polyatomic ions are groups of atoms that have a net electrical charge and behave as a single unit. When naming molecular compounds containing polyatomic ions, the name of the polyatomic ion is used as a single word, and the name of the other element is modified to indicate the number of atoms present.
3. Properties of Molecular Compounds
Molecular compounds generally have lower melting and boiling points compared to ionic compounds. This is because the intermolecular forces between molecules are weaker than the electrostatic forces between ions. The strength of intermolecular forces depends on the polarity of the molecule and the molecular weight.
Polarity refers to the uneven distribution of electrons within a molecule. Polar molecules have a positive end and a negative end, which can result in dipole-dipole interactions. Nonpolar molecules have an even distribution of electrons and do not have a permanent dipole.
4. Chemical Reactions of Molecular Compounds
Molecular compounds can undergo various types of chemical reactions, including combustion, substitution, and addition reactions. Combustion reactions involve the reaction of a hydrocarbon with oxygen, releasing carbon dioxide and water as products.
Substitution reactions involve the replacement of one atom or group of atoms in a molecule with another atom or group of atoms. Addition reactions involve the addition of an atom or group of atoms to a molecule.
The reactivity of molecular compounds depends on the molecular structure and the presence of functional groups. Functional groups are specific arrangements of atoms within a molecule that give the molecule characteristic chemical properties.
5. Applications of Molecular Compounds
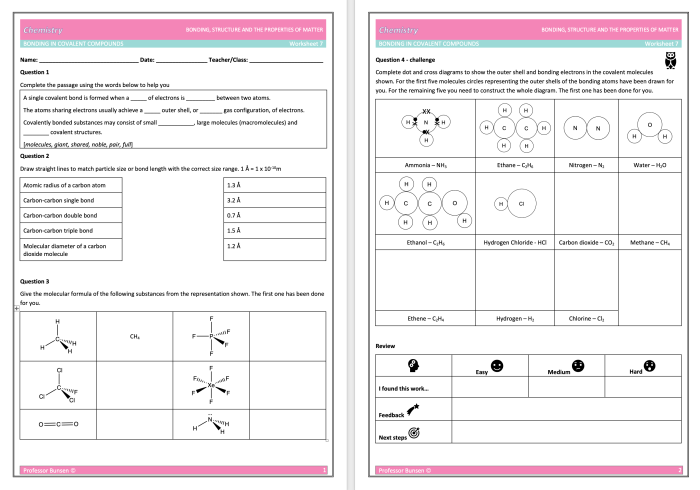
Molecular compounds are essential in everyday life and have a wide range of applications. Examples include:
- Water (H2O): essential for life and used in various industries
- Carbon dioxide (CO2): used in fire extinguishers and as a refrigerant
- Methane (CH4): used as a fuel and in the production of chemicals
- Ethylene (C2H4): used in the production of plastics
- Ethanol (C2H5OH): used as a solvent, fuel, and in alcoholic beverages
Molecular compounds are also crucial in biological systems. For example, DNA and RNA are molecular compounds that carry genetic information, and proteins are molecular compounds that perform various functions in cells.
FAQ Overview
What are the key characteristics of molecular compounds?
Molecular compounds are composed of two or more nonmetallic elements that are covalently bonded. They typically exist as discrete molecules, have relatively low melting and boiling points, and exhibit varying degrees of polarity.
How are molecular compounds named?
Binary molecular compounds are named using prefixes to indicate the number of atoms of each element present. For larger molecules, Greek prefixes are employed. Polyatomic ions are named as a single unit and included in the compound name.
What factors influence the physical properties of molecular compounds?
The physical properties of molecular compounds, such as melting and boiling points, are primarily determined by the strength of the intermolecular forces present. These forces include dipole-dipole interactions, hydrogen bonding, and London dispersion forces.