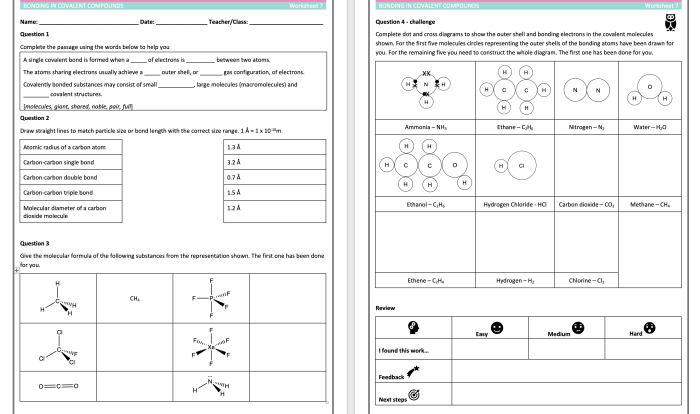
Welcome to the comprehensive naming ionic compounds practice worksheet, a meticulously crafted resource designed to guide you through the intricacies of ionic compound nomenclature. As you embark on this journey, you will delve into the systematic rules that govern the naming of these essential chemical entities, empowering you with the knowledge to decipher and construct their intricate chemical identities.
Throughout this worksheet, you will encounter a diverse array of practice problems that challenge your understanding of ionic compound naming. Engage with these exercises to reinforce your grasp of cation and anion nomenclature, unravel the complexities of variable charge metal cations, and navigate the nuances of naming compounds with both monoatomic and polyatomic ions.
Immerse yourself in the captivating world of ionic compounds and emerge as a master of their nomenclature.
Ionic Compounds Nomenclature Principles

Ionic compounds are formed when a metal loses one or more electrons to a nonmetal. The resulting ions have opposite charges and are attracted to each other by electrostatic forces. The naming of ionic compounds follows specific rules that ensure consistency and clarity in chemical communication.
Cation and Anion Naming
- Cationsare positively charged ions formed when a metal loses electrons. The name of a cation is simply the name of the metal followed by the suffix “-ium”. For example, Na +is sodium ion, Ca 2+is calcium ion, and Al 3+is aluminum ion.
- Anionsare negatively charged ions formed when a nonmetal gains electrons. The name of an anion depends on the number of electrons gained. If the nonmetal gains one electron, the anion name ends in “-ide”. If the nonmetal gains two electrons, the anion name ends in “-ite”.
If the nonmetal gains three electrons, the anion name ends in “-ate”. For example, Cl –is chloride ion, O 2-is oxide ion, and N 3-is nitride ion.
Binary Ionic Compounds, Naming ionic compounds practice worksheet
Binary ionic compounds are composed of a metal cation and a nonmetal anion. To name a binary ionic compound, simply combine the name of the cation with the name of the anion. For example, NaCl is sodium chloride, CaO is calcium oxide, and AlN is aluminum nitride.
Roman Numerals for Variable Charge Metal Cations
Some metal cations can have multiple charges. To distinguish between these different charges, Roman numerals are used in the cation name. The Roman numeral indicates the charge of the cation. For example, Fe 2+is iron(II) ion and Fe 3+is iron(III) ion.
Practice Exercises
To practice naming ionic compounds, complete the following worksheet:
- Name the following ionic compounds:
- Write the formulas for the following ionic compounds:
| Formula | Name |
|---|---|
| KCl | |
| CaO | |
| AlN | |
| FeCl3 | |
| CuO |
| Name | Formula |
|---|---|
| Sodium chloride | |
| Calcium oxide | |
| Aluminum nitride | |
| Iron(III) chloride | |
| Copper(II) oxide |
Advanced Naming Considerations

The naming of ionic compounds becomes more complex when dealing with polyatomic ions and transition metal cations.
Naming Ionic Compounds with Complex Anions
Polyatomic ions are ions that contain more than one atom. The naming of polyatomic ions follows specific rules. For example, the sulfate ion (SO 42-) is named by combining the root name of the central atom (sulfur) with the suffix “-ate”.
The prefix “per-” is used to indicate that the anion contains the maximum number of oxygen atoms possible. For example, the perchlorate ion (ClO 4–) is named by combining the root name of the central atom (chlorine) with the suffix “-ate” and the prefix “per-“.
Naming Ionic Compounds with Transition Metal Cations
Transition metal cations can have multiple oxidation states. To indicate the oxidation state of the transition metal cation, Roman numerals are used in the compound name. For example, the copper(II) ion (Cu 2+) is named by combining the root name of the metal (copper) with the suffix “-ous” and the Roman numeral II to indicate the oxidation state.
Visual Aids and Illustrations: Naming Ionic Compounds Practice Worksheet
The following table illustrates the relationship between the charge of an ion and its name:
| Charge | Name |
|---|---|
| +1 | -ium |
| +2 | -ous |
| +3 | -ic |
| -1 | -ide |
| -2 | -ite |
| -3 | -ate |
The following flowchart guides students through the steps of naming ionic compounds:
- Identify the metal and nonmetal ions in the compound.
- Name the cation by adding the suffix “-ium” to the name of the metal.
- Name the anion by adding the suffix “-ide”, “-ite”, or “-ate” to the root name of the nonmetal.
- Combine the names of the cation and anion to form the name of the ionic compound.
The following illustrations show the structures of various ionic compounds:
- Sodium chloride (NaCl):

- Calcium oxide (CaO):

- Aluminum nitride (AlN):

Query Resolution
What is the primary objective of this naming ionic compounds practice worksheet?
This worksheet aims to provide a comprehensive practice experience in naming ionic compounds, enhancing your understanding of the systematic rules governing their nomenclature.
How does this worksheet cater to different levels of learners?
The practice problems are designed to accommodate a range of learning levels, from beginners seeking to grasp the basics to advanced learners looking to refine their skills in naming complex ionic compounds.
What resources are available within this worksheet to aid in learning?
In addition to the practice problems, the worksheet offers clear explanations of the underlying principles of ionic compound nomenclature, ensuring a thorough understanding of the concepts.