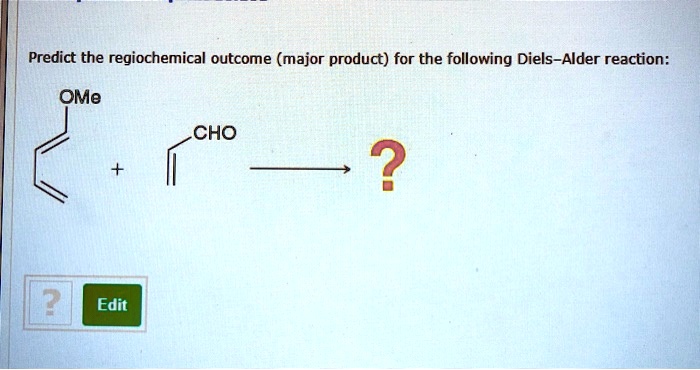
Predict the product of the following Diels-Alder reaction, a cornerstone in organic chemistry. This reaction, characterized by its cycloaddition mechanism, holds immense significance in synthesizing complex organic molecules. Delving into the intricacies of this reaction, we will explore the factors influencing product formation, regioselectivity, stereoselectivity, and its myriad applications.
The Diels-Alder reaction, a powerful tool in the organic chemist’s arsenal, offers a versatile platform for constructing cyclic compounds. Understanding the factors governing product formation is crucial for harnessing the full potential of this reaction. Substituents on the diene and dienophile, along with reaction conditions, play a pivotal role in shaping the outcome of the reaction.
Introduction
Diels-Alder reactions are a type of cycloaddition reaction that involves the reaction of a conjugated diene with a dienophile to form a cyclohexene ring. These reactions are highly stereoselective and regiospecific, making them a powerful tool for the synthesis of complex organic molecules.
The mechanism of the Diels-Alder reaction proceeds through a concerted [4+2] cycloaddition, in which the two π-bonds of the diene and the two π-bonds of the dienophile react simultaneously to form the cyclohexene ring. The reaction is exothermic and proceeds with high regio- and stereoselectivity.
Predicting the product of a Diels-Alder reaction is essential for planning and executing organic synthesis. The regioselectivity of the reaction is determined by the relative reactivity of the diene and dienophile, while the stereoselectivity is determined by the orientation of the diene and dienophile in the transition state.
Factors Influencing Product Formation
The product of a Diels-Alder reaction is determined by several factors, including the structure of the diene and dienophile, the reaction conditions, and the presence of substituents.
The diene must be conjugated in order to undergo a Diels-Alder reaction. The more conjugated the diene, the more reactive it will be. The dienophile must also be able to accept two electrons. Dienophiles that are electron-deficient are more reactive than those that are electron-rich.
The reaction conditions can also affect the product of a Diels-Alder reaction. The reaction is typically carried out in a solvent such as dichloromethane or toluene. The temperature of the reaction can also affect the regio- and stereoselectivity of the reaction.
Substituents on the diene and dienophile can also affect the product of a Diels-Alder reaction. Electron-donating substituents on the diene will increase its reactivity, while electron-withdrawing substituents will decrease its reactivity. Electron-withdrawing substituents on the dienophile will increase its reactivity, while electron-donating substituents will decrease its reactivity.
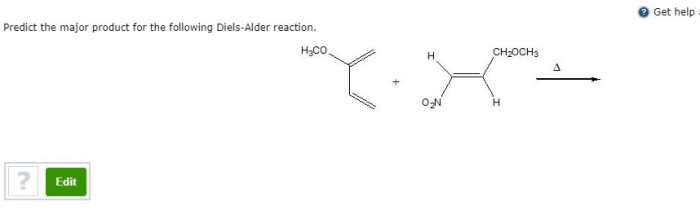
Regioselectivity and Stereoselectivity, Predict the product of the following diels-alder reaction
The regioselectivity of a Diels-Alder reaction is determined by the relative reactivity of the diene and dienophile. The more reactive the diene, the more likely it is to react with the dienophile. The more reactive the dienophile, the more likely it is to react with the diene.
The stereoselectivity of a Diels-Alder reaction is determined by the orientation of the diene and dienophile in the transition state. The most stable transition state is the one in which the diene and dienophile are oriented in a way that minimizes steric hindrance.
This transition state leads to the formation of the most stable product.
Applications of Diels-Alder Reactions
Diels-Alder reactions are a powerful tool for the synthesis of complex organic molecules. They are used in the synthesis of a wide variety of natural products and pharmaceuticals. Some of the most common applications of Diels-Alder reactions include:
- The synthesis of steroids
- The synthesis of alkaloids
- The synthesis of terpenes
- The synthesis of pharmaceuticals
Diels-Alder reactions are a versatile and powerful tool for the synthesis of complex organic molecules. They are used in a wide variety of applications, including the synthesis of natural products, pharmaceuticals, and other organic compounds.
Questions Often Asked: Predict The Product Of The Following Diels-alder Reaction
What is the Diels-Alder reaction?
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a cyclic compound.
What factors influence the regioselectivity of the Diels-Alder reaction?
The regioselectivity of the Diels-Alder reaction is influenced by the electronic and steric effects of substituents on the diene and dienophile.
What is stereoselectivity in the Diels-Alder reaction?
Stereoselectivity in the Diels-Alder reaction refers to the preferential formation of one stereoisomer over another.