With the periodic table worksheet answers at the forefront, this comprehensive guide delves into the fascinating world of chemistry, offering a captivating exploration of the elements and their properties. Through an engaging journey, readers will uncover the secrets of the periodic table, unlocking a wealth of knowledge that shapes our understanding of the universe.
From its humble beginnings to its profound impact on modern science, the periodic table stands as a testament to the human quest for knowledge. Its organization, based on atomic number and electron configuration, provides a systematic framework for understanding the behavior and interactions of elements.
This guide will delve into the key features of the periodic table, exploring the periodic trends, group and period relationships, and chemical reactivity of the elements.
Periodic Table Worksheet Answers: Overview: The Periodic Table Worksheet Answers
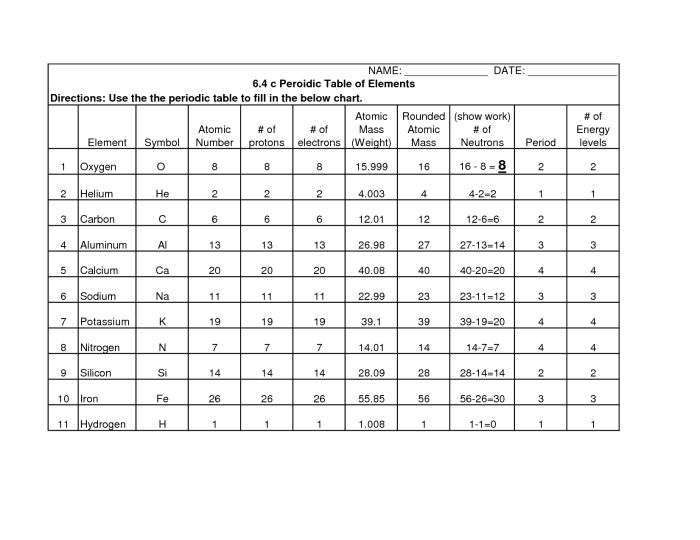
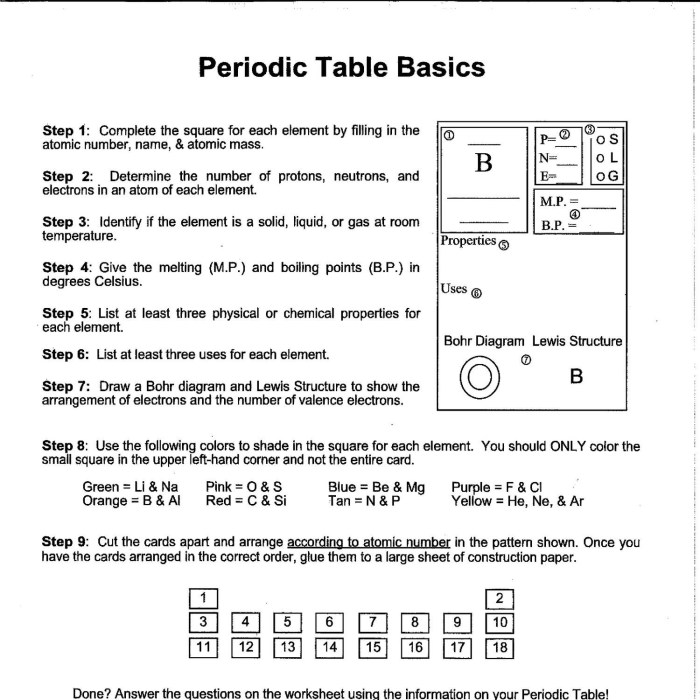
A periodic table worksheet is a valuable tool for students to learn and practice their understanding of the periodic table.
The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic number, electron configurations, and recurring chemical properties. It is generally accepted that the modern periodic table was first published by Dmitri Mendeleev in 1869, although several other scientists had developed similar tables prior to this.
The periodic table is a powerful tool for organizing and understanding the vast array of chemical elements. It can be used to predict the properties of an element based on its position in the table, and to understand the relationships between different elements.
Element Properties and Trends
| Element Symbol | Atomic Number | Atomic Mass | Group | Period | Electron Configuration |
|---|---|---|---|---|---|
| H | 1 | 1.008 | 1 | 1 | 1s1 |
| He | 2 | 4.0026 | 18 | 1 | 1s2 |
| Li | 3 | 6.941 | 1 | 2 | 1s2 2s1 |
| Be | 4 | 9.0122 | 2 | 2 | 1s2 2s2 |
| B | 5 | 10.811 | 13 | 2 | 1s2 2s2 2p1 |
The periodic table can be used to identify trends in the properties of the elements. For example, the atomic radius of the elements generally increases down a group and decreases across a period. This is because the atomic radius is determined by the number of electron shells, and the number of electron shells increases down a group and decreases across a period.
Group and Period Relationships, The periodic table worksheet answers
The elements in the periodic table are grouped together based on their chemical properties. The elements in each group have similar valence electron configurations, which gives them similar chemical properties. The elements in each period have the same number of electron shells, which gives them similar physical properties.
For example, the elements in Group 1 (the alkali metals) are all highly reactive metals. The elements in Group 18 (the noble gases) are all non-reactive gases.
Chemical Reactions and Reactivity
The periodic table can be used to predict the chemical reactions that an element will undergo. The reactivity of an element is determined by its position in the periodic table. The elements in the lower left-hand corner of the periodic table are the most reactive, while the elements in the upper right-hand corner of the periodic table are the least reactive.
For example, the alkali metals (Group 1) are highly reactive and will react with water to produce hydrogen gas. The noble gases (Group 18) are non-reactive and will not react with any other elements.
Applications of the Periodic Table
The periodic table is a valuable tool for scientists and engineers. It is used in a wide variety of applications, including:
- Predicting the properties of new elements
- Designing new materials
- Understanding the chemical reactions that occur in nature
- Developing new technologies
The periodic table is a powerful tool that has helped us to understand the world around us. It is a testament to the human intellect and a valuable resource for scientists and engineers.
FAQ Section
What is the purpose of a periodic table worksheet?
Periodic table worksheets provide a structured framework for students to learn and practice identifying and understanding the properties of elements based on their position in the periodic table.
How can the periodic table be used to predict chemical reactions?
The periodic table can be used to predict the reactivity and behavior of elements in chemical reactions based on their position and trends within the table, such as electronegativity and ionization energy.